 |
Isolation and purification. Fermentation scale-up. Enzyme technology
|
|
|
|
Isolation and purification
Crude broth produced by fermentation requires further isolation and purification for recovering the desired product in a suitably pure state. Downstream processes typically consist of a series of steps that eventually provide the product at the desired level of purity.
The extraction and purification of fermentation products may be difficult and costly. Product recovery and purification often contribute 70 – 80% to the final cost of producing a product. Therefore, the design and efficient operation of these processes are vital elements in getting the required products into commercial use, and should reflect the need not to lose more of the desired product than is absolutely necessary.
The product may be at a low concentration in an aqueous solution that contains intact microorganisms, cell fragments, soluble and insoluble medium components and other metabolic products. The product may also be intracellular, heat labile and easily broken down by contaminating microorganisms. The processing equipment must therefore be of the correct type and also the correct size to ensure that the harvested broth can be processed within a satisfactory time limit.
The extraction and purification include the following operations:
- Separation (filtration, centrifugation, flotation, disruption)
- Concentration (solubilisation, extraction, thermal processing, membrane filtration, precipitation)
- Purification (crystallization, chromatography)
- Modification
- Drying
Many products cannot be supplied easily in a dried form and must be sold in liquid preparations. Care must be taken to avoid microbial contamination and deterioration and, when the product is proteinaceous, to avoid denaturation.
When the product has been separated from the liquid phase and the solid phase (e. g. microbial biomass), the broth will be disposed of by standard sewage treatment.
Fermentation scale-up
An important aspect of fermentation technology is the transfer of a process from small-scale laboratory equipment to large-scale commercial equipment. It is called scale-up. An understanding of the problems in scale-up is very important because bio catalytic processes do not have the same behavior in small scale and in large scale. Scale-up requires knowledge not only of the biology of the producing organism but also of the physics of bioreactor design and operation. Aeration and mixing are very important. Their behavior is easier in small laboratory flask than in large industrial bioreactors.
Oxygen transfer specially is much more difficult to obtain in a large bioreactor, and because most of industrial fermentations are aerobic, effective oxygen transfer is essential. If concentration of biomass is high the oxygen demand is too high, then a good aeration is required to achieve high product yield.
Transferring an industrial process from the laboratory to commercial bioreactor involves several stages. Things begin in the laboratory flask, a very small-scale operation but typically the first indication that a process of commercial interest is possible. From here, things are transferred to the laboratory bioreactor, a small-scale bioreactor, generally of glass and of 1 to 10 L size, in which the first efforts at scale-up are made. In the laboratory bioreactor, it is possible to test variations in medium, temperature, pH, and so on, inexpensively because little cost is involved for either equipment or culture medium.
|
|
|
When tests in the laboratory bioreactor are successful, the process moves into the pilot plant stage, usually carried out in equipment 300-3000 L in size. Here the conditions more closely approach the commercial scale; however, cost is not yet a major factor. Finally, the process is moved to the commercial bioreactor itself, typically 10 000-500 000 L in volume.
In all stages, aeration is very closely monitored. As scale-up proceeds from flask to production bioreactor, oxygen dynamics are carefully measured at each step to determine how volume increases affect oxygen demand in the fermentation.
The management of scale-up requires high capital investment in mixing and aeration, in monitoring and control devices, and in stringent maintenance of sterility.
Enzyme technology
As you could understand, we discussed the most important aspects of bioprocess technology and the main steps of fermentation process. We can summarize, that in bioprocess a large number of cells grows in the bioreactor at controlled conditions to get a desired product with defined quality parameters, considering the highest efficiency.
The enzyme technology is a very important area of bioprocesses. It belong to one of the main areas of Biotechnology that has permitted the solution of numerous health and environmental problems in the industrialized world.
For centuries, man has used microorganisms to his advantage without knowing that the transformations obtained were due to the function of certain enzymes present in the organisms used. Thus, before the biochemical bases of the biocatalyzed processes were known, the catalytic capacity of the enzymes present in microorganisms such as Saccharomyces cerevisiae yeast or lactic bacteria have been used for centuries in the production of foods such as wine, beer, cheese, vinegar or bread.
Although the term enzyme was first coined by Kü ne in 1876, deep knowledge of enzymes, their structure, kinetics, structure-function relationship, has not been achieved until recent years, when enzyme research acquires a new phase thanks to the knowledge contributed from different disciplines such as Protein chemistry, Biophysics and Molecular biology.
At present, there are numerous biotechnological processes that are carried out using cells or their isolated enzymes, being a field with great future perspectives.
Biocatalysis has emerged as an area of great wealth within Biotechnology, and has allowed the application of enzymes in a large number of industries dedicated to the manufacture of drugs and other chemical compounds, as well as food or biofuels, or in the textile and detergent industry, among many other examples. This is due to the fact that they represent a more efficient and at the same time more ecological alternative to traditional synthetic chemistry, since the processes they catalyze pass through reactions in more friendly environments and under mild pH and temperature conditions with higher yields. It is not surprising that the enzyme business has grown steadily in recent years, and expectations indicate that the global market can be in figures above $ 4400 million in the coming years.
|
|
|
The term Biocatalysis refers to the use of cells or their isolated enzymes to catalyze reactions or transformations that lead to obtaining compounds of interest, which meet numerous human needs. Biocatalysis is framed in the so-called White Biotechnology, subsector of Biotechnology with important growth prospects, key to the development of the so-called bioeconomy.
What are enzymes and what role do they play?
Enzymes are a special class of proteins that accelerate the speed of chemical reactions that occur in a cell. This is why they are known as " biological catalysts". They show very interesting properties for the industry: they are highly specific, work in mild conditions, are easily available and preserve the environment.
Nomenclature
The nomenclature of enzymes is derived from their substrates or the catalyzed chemical reactions, and " ase" is usually added as a suffix. Enzymes can be indexed with letters and numbers according to International Union of Biochemistry and Molecular Biology: the letter EC plus four numbers representing four elements. The first number represents enzymes that are classified according to the mechanism of enzymatic reaction.
Classification
According to the type of reactions that the enzymes catalyze, enzymes are classified into six categories:
ü Oxidoreductases
ü Transferases
ü Hydrolases
ü Lyases
ü Isomerases
ü Ligases
Oxidoreductases, transferases and hydrolases are the most abundant forms of enzymes. Individual enzyme classes are further classified systematically based on the chemical name of the substrate and its reaction mechanism (Table 1).
Table 1. Classification of the enzymes based on the chemical name of the substrate and its reaction mechanism.
| Enzyme class | Reaction type | Description |
| EC 1 Oxidoreductases | 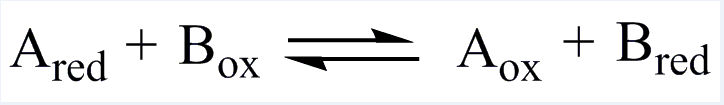
| Catalyze redox reaction and can be categorized into oxidase and reductase. |
| EC 2 Transferases | 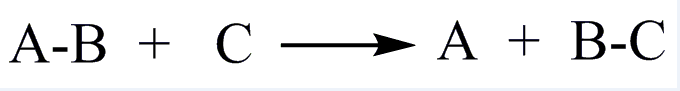
| Catalyze the transfer or exchange of certain groups among some substrates |
| EC 3 Hydrolases | 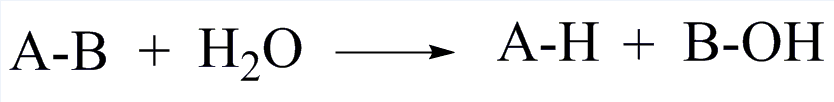
| Accelerate the hydrolysis of substrates |
| EC 4 Lyases | 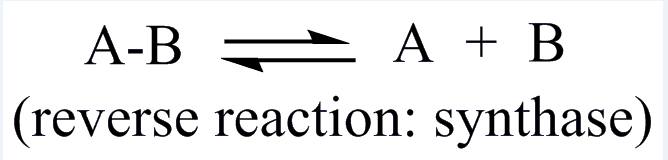
| Promote the removal of a group from the substrate to leave a double bond reaction or catalyze its reverse reaction |
| EC 5 Isomerases | 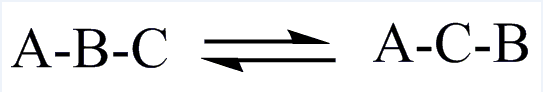
| Facilitate the conversion of isoisomers, geometric isomers or optical isomers. |
| EC 6 Ligases | 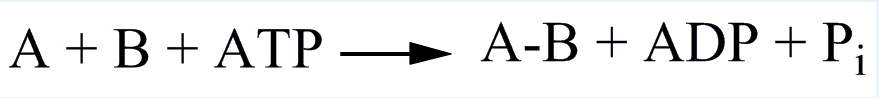
| Catalyze the synthesis of two molecular substrates into one molecular compound with the release energy |
According to the unified classification principle of enzymes published by the International Society of Biochemistry, each group of enzymes in the above six categories can be further divided into several subgroups according to the characteristics of the functional groups or bonds in the substrates. In order to show the properties of substrates or reactants more accurately, each subclass is further divided into subclasses and directly contains a quantity of enzymes.
Moreover, based on the molecular composition, enzymes can be divided into pure enzymes and binding enzymes. Enzymes containing only protein are called pure enzymes. Binding enzymes are composed of proteins and cofactors. Only when the two components are combined, can the enzyme have catalytic activity.
Mode of action
In an enzyme catalyzed reaction (E), the reagents are called substrates (S), that is, the substance on which the enzyme acts. The substrate is chemically modified and becomes one or more products (P). As this reaction is reversible, it is expressed as follows:
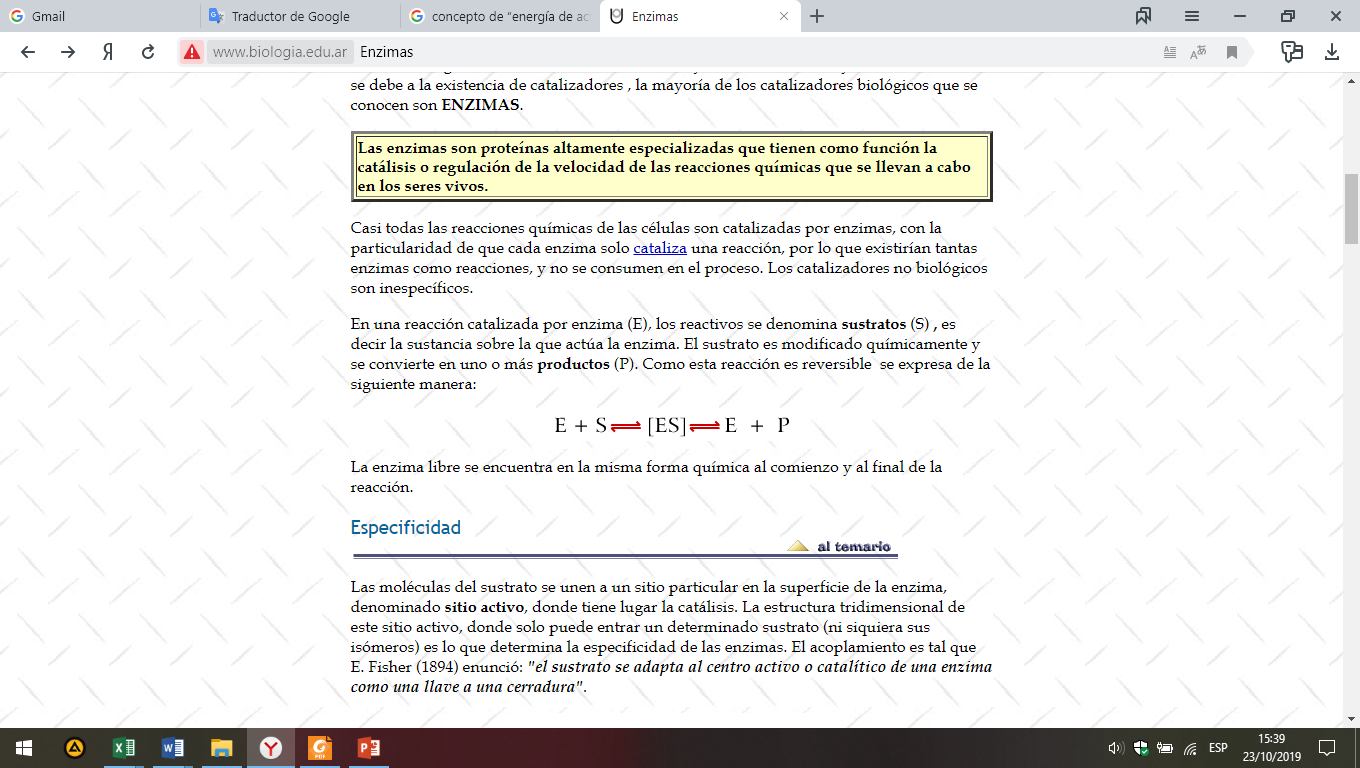
The free enzyme is in the same chemical form at the beginning and at the end of the reaction.
|
|
|
The mode of action is specific since each type of enzyme acts on a particular type of reaction and on a specific substrate.
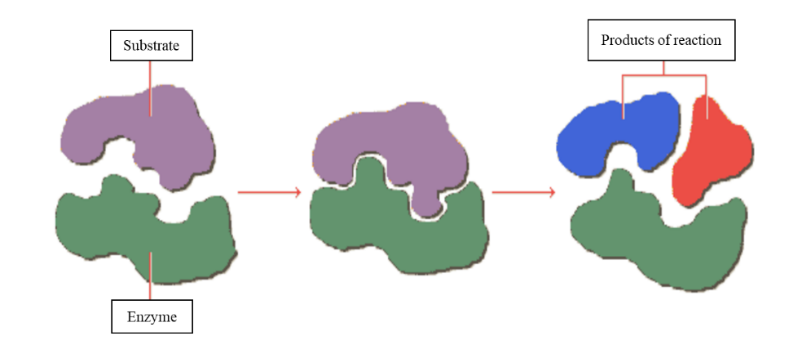
Fig. 5. The enzyme mode of action.
To perform its function, an enzyme recognizes a specific molecule, called a substrate. Each enzyme binds to its specific substrate at the active site (Fig. 5) and causes a chemical change in it, whereby a product is obtained. The change involves the formation or breakage of a covalent bond. The enzyme that participates in the reaction does not undergo modifications, and can act again on another substrate of the same type. In the absence of enzymes, biochemical reactions would be extremely slow and life would not be possible. Enzymes can increase the speed of reactions by a million times.
The substrate to product reaction does not occur spontaneously since energy is needed (called activation energy). The enzyme decreases the activation energy necessary for the reaction to occur spontaneously, making the process faster. Fig. 6 shows this behavior.
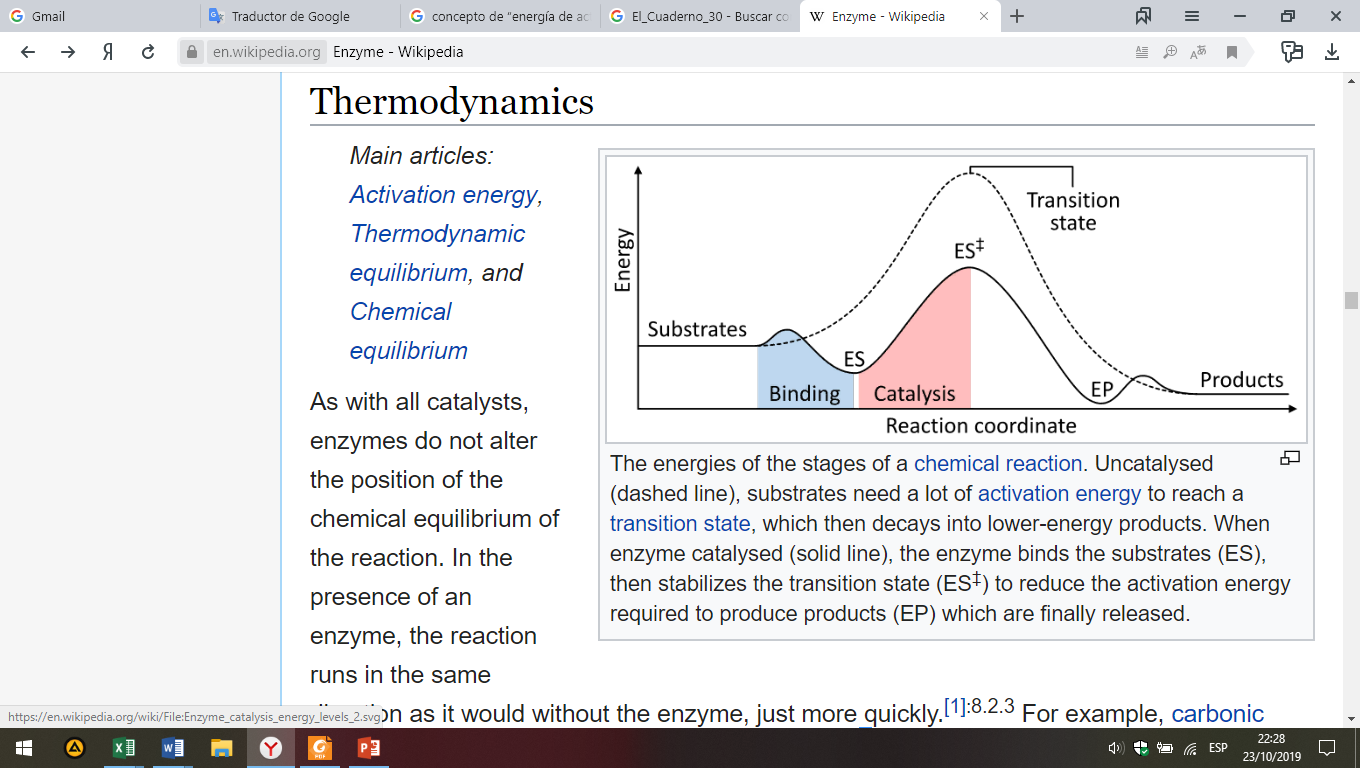
Fig. 6. Energies of the different phases of a chemical reaction.
Uncatalysed (dashed line), substrates need a lot of activation energy to reach a transition state, which then decays into lower-energy products. When enzyme catalysed (solid line), the enzyme binds the substrates (ES), then stabilizes the transition state (ES‡) to reduce the activation energy required to produce products (EP) which are finally released.
As with all catalysts, enzymes do not alter the position of the chemical equilibrium of the reaction. In the presence of an enzyme, the reaction runs in the same direction as it would without the enzyme, just more quickly.
Factors affecting catalytic activity of enzymes
The rate of an enzyme-catalyzed reaction depends on the concentrations of enzyme and substrate. As the concentration of both is increased the rate of reaction increases.
For a given enzyme concentration, the rate of reaction increases with increasing substrate concentration up to a point, above which any further increase in substrate concentration produces no significant change in reaction rate. This is because the active sites of the enzyme molecules at any given moment are virtually saturated with substrate. The enzyme/substrate complex has to dissociate before the active sites are free to accommodate more substrate.
Through the enzyme kinetics was investigated how enzymes bind substrates and turn them into products. The rate data used in kinetic analyses are commonly obtained from enzyme assays. In 1913, Leonor Michaelis and Maud Leonora Menten proposed a quantitative theory of enzyme kinetics, which is referred to as Michaelis–Menten kinetics. The major contribution of Michaelis and Menten was to think of enzyme reactions in two stages (Fig. 7). In the first, the substrate binds reversibly to the enzyme, forming the enzyme-substrate complex. This is sometimes called the Michaelis-Menten complex in their honor. The enzyme then catalyzes the chemical step in the reaction and releases the product. This work was further developed by G. E. Briggs and J. B. S. Haldane, who derived kinetic equations that are still widely used today.
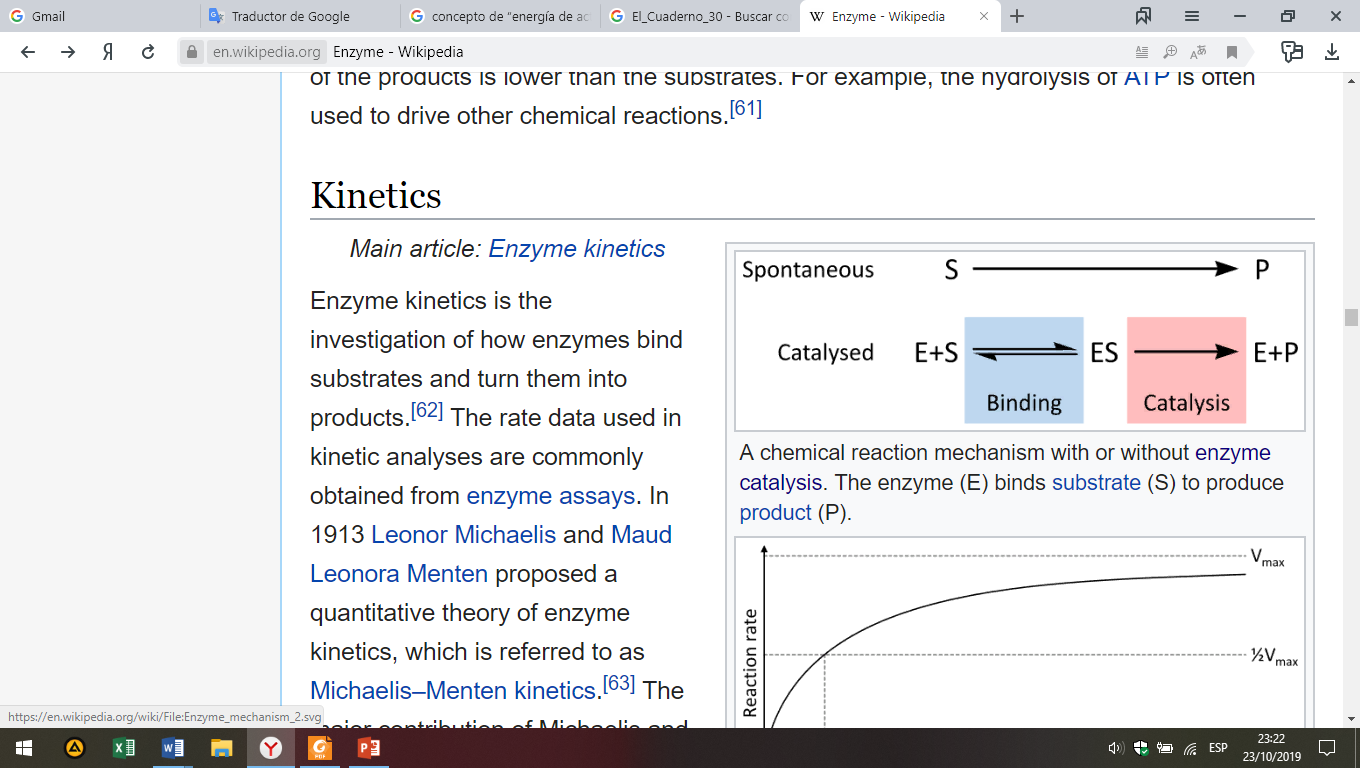
Fig. 7. A chemical reaction mechanism with or without enzyme catalysis. The enzyme (E) binds substrate (S) to produce product (P).
Enzyme rates depend on solution conditions and substrate concentration. To find the maximum speed of an enzymatic reaction, the substrate concentration is increased until a constant rate of product formation is seen. This is shown in the saturation curve in Fig. 8. Saturation happens because, as substrate concentration increases, more and more of the free enzyme is converted into the substrate-bound ES complex. At the maximum reaction rate (Vmax) of the enzyme, all the enzyme active sites are bound to substrate, and the amount of ES complex is the same as the total amount of enzyme.
|
|
|
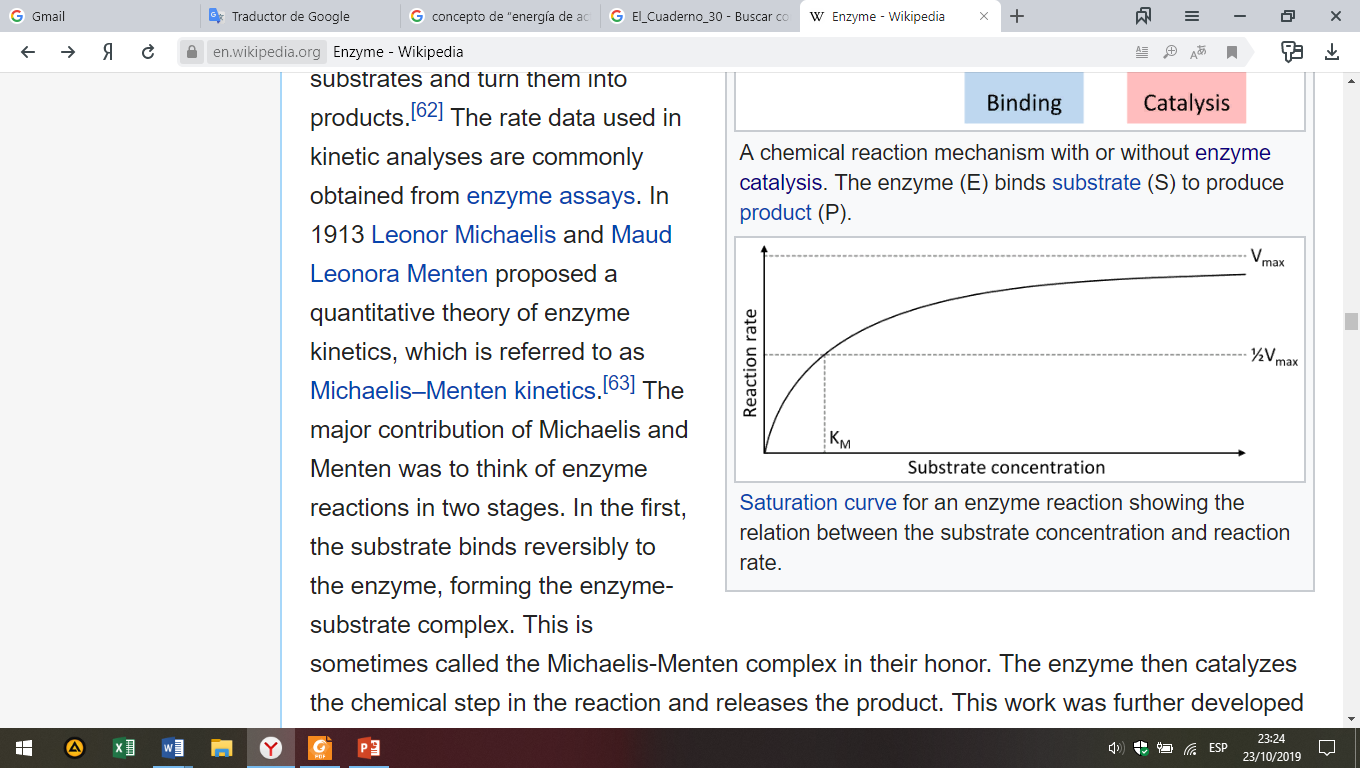
Fig. 8. Saturation curve for an enzyme reaction.
Vmax is only one of several important kinetic parameters. The amount of substrate needed to achieve a given rate of reaction is also important. This is given by the Michaelis-Menten constant (KM), which is the substrate concentration required for an enzyme to reach one-half its maximum reaction rate; generally, each enzyme has a characteristic KM for a given substrate. Another useful constant is kcat, also called the turnover number, which is the number of substrate molecules transformed into product per unit of time per catalyst molecule.
The efficiency (catalytic efficiency is a measure of how efficiently an enzyme converts a substrate into product) of an enzyme can be expressed in terms of kcat /KM. This is also called the specificity constant and incorporates the rate constants for all steps in the reaction up to and including the first irreversible step. Because the specificity constant reflects both affinity and catalytic ability, it is useful for comparing different enzymes against each other, or the same enzyme with different substrates. The theoretical maximum for the specificity constant is called the diffusion limit and is about 108 to 109 (mol s− 1). At this point every collision of the enzyme with its substrate will result in catalysis, and the rate of product formation is not limited by the reaction rate but by the diffusion rate. Enzymes with this property are called catalytically perfect or kinetically perfect.
Like all proteins, enzymes are very sensitive to changes in temperature and acidity.
Each type of enzyme works optimally at a certain temperature; if the temperature of the medium in which the enzyme is located is far from optimal, the enzyme decreases its activity. This particularity of enzymes can be represented in Fig. 9:
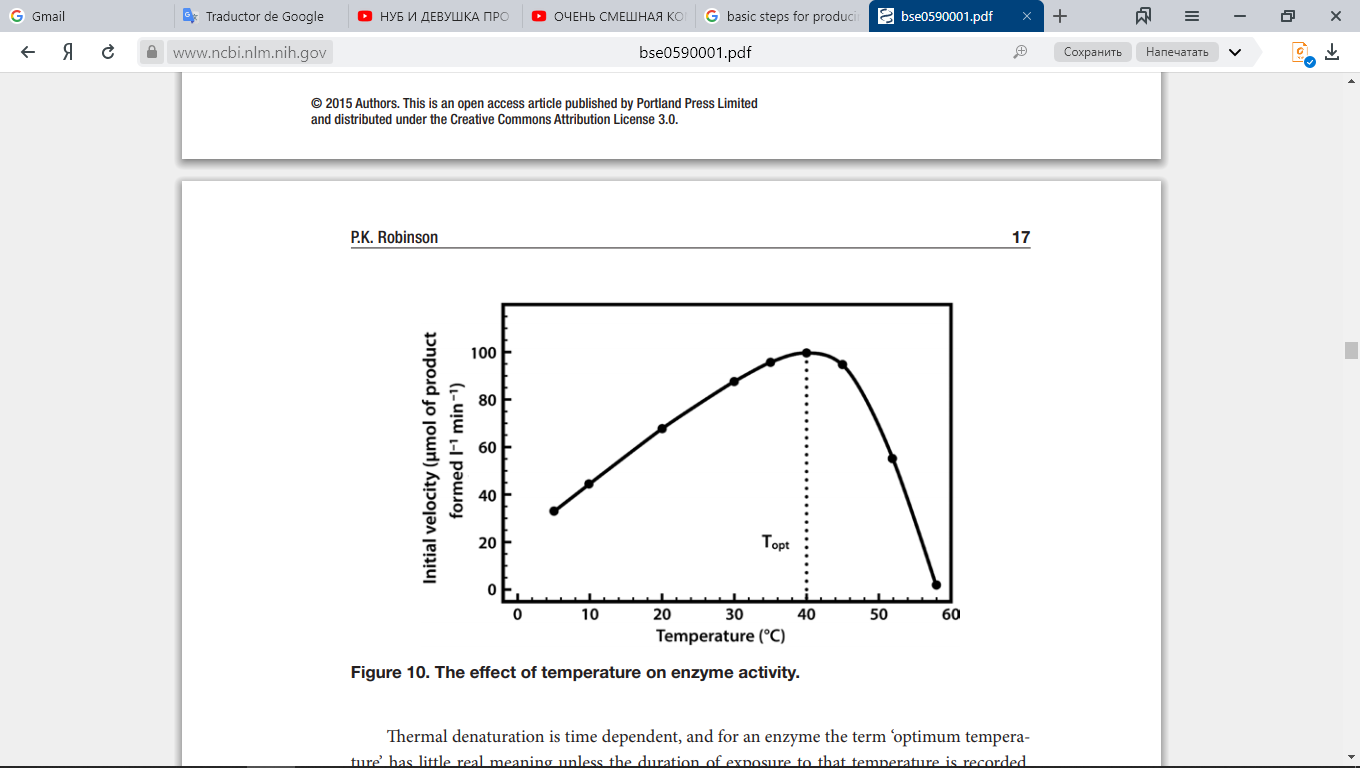
Fig. 9. Enzymatic activity as a function of temperature.
The graph shows how enzyme activity varies (expressed in percentages) as the temperature rises. The optimal temperature of this enzyme is 40 °C. At that temperature, the enzyme works at 100%. At temperatures below and above 40 °C the curve descends, which shows a decrease in the activity of the enzyme. Below the optimum temperature, the enzymatic activity decreases because the mobility of the molecule is reduced, while at temperatures above the optimum the reduction in activity is attributed to denaturation.
Similarly, each enzyme works within quite a small pH range, as is shown in Fig. 10. There is a pH at which its activity is greatest (the optimal pH). This is because changes in pH can make and break intra- and intermolecular bonds, changing the shape of the enzyme and, therefore, its effectiveness.
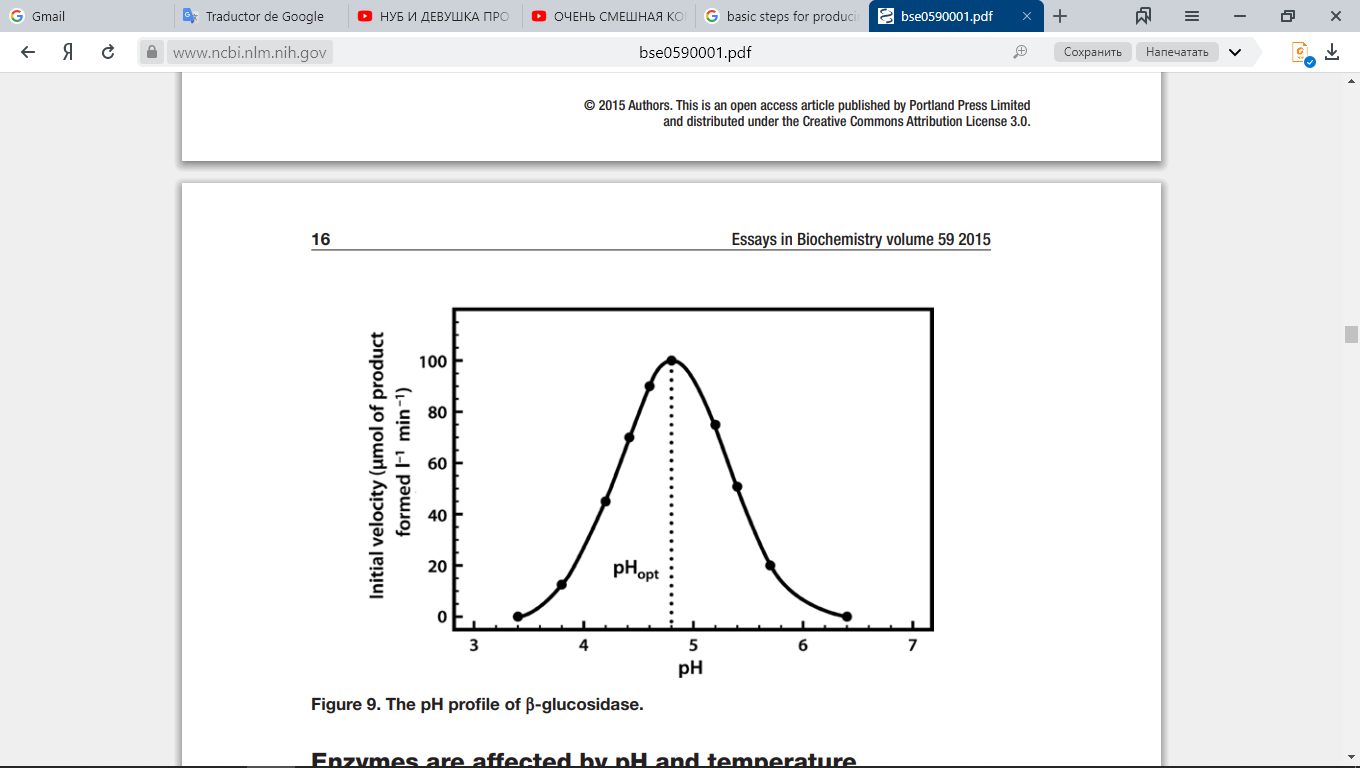
Fig. 10. Enzymatic activity as a function of pH.
Control questions:
1. Why Industrial Microbiology is important?
2. Mention the main characteristics of an industrial microorganism.
3. Explain why ethanol is a primary metabolite.
4. Indicate some environmental factors that should be controlled during fermentation process. Explain how the variation of one of them can affect the process.
5. Discuss scale-up and the importance of fermentation scale-up.
6. Mention some examples of microbial products.
7. Define the biocatalytic process and explain its importance.
8. What is the nature of an enzyme? Explain its mode of action. Consider the activation energy and the most important parameters on which the enzymatic activity depends.
Suggested literature
-Smith J. E. Biotechnology. Fifth Edition, 2009, 280 p. ISBN-13 978-0-511-46394-5
- Stanbury P. F., Whitaker A., Hall S. J. Principles of fermentation technology. 2nd Edition, 1995, Elsevier Science Ltd., 367 p. ISBN 0 7506 4501 6.
- Robinson P. K. Enzymes: principles and biotechnological applications. Essays Biochem. (2015) 59, 1–
41: doi: 10. 1042/BSE0590001.
Next time we will continue studying the enzyme technology as a very important area of Biotechnology that has permitted the solution of numerous health and environmental problems in the industrialized world.
|
|
|


