 |
Full size image
|
|
|
|
Full size image
Mitochondrial damage
Mitochondria are energy production centres involved in various signalling pathways in cells and are also a key point of apoptotic regulation [83]. After exposure to GO and carboxyl graphene (GXYG), the mitochondrial membrane was depolarized, and the amount of mitochondria decreased in HepG2 cells [180]. Exposure to GFNs resulted in significantly increased coupled and uncoupled mitochondrial oxygen consumption, dissipation of the mitochondrial membrane potential, and eventual triggering of apoptosis by activating the mitochondrial pathway [181]. For instance, GO increased the activity of mitochondrial electron transport complexes I/III and the supply of electrons to site I/II of the electron transport chain, accelerating the generation of ROS during mitochondrial respiration in MHS cells [99]. The formation of •OH mediated by GO and the cytochrome-c/H2O2 electron-transfer system could enhance oxidative and thermal stress to impair the mitochondrial respiration system and eventually result in dramatic toxicity [151]. Additionally, the oxygen moieties on GO might accept electrons from cellular redox proteins, supporting the redox cycling of cytochrome c and electron transport proteins, and cytochromes MtrA, MtrB, and MtrC/OmcA might be involved in transferring electrons to GO [182]. Therefore, except for the plasma membrane damage and oxidative stress induction, GFNs can cause apoptosis and/or cell necrosis by direct influencing cell mitochondrial activity [183, 184].
DNA damage
Due to its small size, high surface area and surface charge, GO may possess significant genotoxic properties and cause severe DNA damage, for example, chromosomal fragmentation, DNA strand breakages, point mutations, and oxidative DNA adducts and alterations [87, 122, 185, 186]. Mutagenesis was observed in mice after intravenous injection of GO at a dose of 20 mg/kg compared with cyclophosphamide (50 mg/kg), a classic mutagen [112]. Even if GO cannot enter into the nucleus of a cell, it may still interact with DNA during mitosis when the nuclear membrane breaks down, which increases the opportunity for DNA aberrations [87, 147, 187, 188]. The π stacking interaction between the graphene carbon rings and the hydrophobic DNA base pairs can make a DNA segment ‘stand up’ or ‘lay on’ the surface of graphene with its helical axis perpendicular or parallel, respectively. The intermolecular forces severely deform the end base pairs of DNA, which potentially increases the genotoxicity [189]. GO may also induce chromosomal fragmentation, DNA adducts and point mutations by promoting oxidative stress or triggering inflammation through the activation of intracellular signalling pathways such as MAPK, TGF-β and NF-κ B [110, 112, 146]. Graphene and rGO can also elevate the expression of p53, Rad51, and MOGG1-1, which reflect chromosomal damage, and decrease the expression of CDK2 and CDK4 by arresting the cell cycle transition from the G1 to the S phase in various cell lines [112]. DNA damage can not only initiate cancer development but also possibly threaten the health of the next generation if the mutagenic potential of GO arises in reproductive cells, which impacts fertility and the health of offspring [112, 190].
|
|
|
Inflammatory response
GFNs can cause a significant inflammatory response including inflammatory cell infiltration, pulmonary edema and granuloma formation at high doses via intratracheally instillation or intravenous administration [30, 49]. Platelets are the important components in clot formation to attack pathogens and particulate matter during the inflammatory response, and GO could directly activate platelet-rich thrombi formation to occlude lung vessels after intravenous injection [98, 191]. A strong inflammatory response was induced by subcutaneously injection with GO for 21 days, along with the secretion of key cytokines, including IL-6, IL-12, TNF-α, MCP-1, and IFN-g [34, 192]. GFNs can trigger an inflammatory response and tissue injury by releasing cytokines and chemokines that lead to the recruitment of circulating monocytes and stimulating the secretion of Th1/Th2 cytokines and chemokines [124, 193]. Additionally, pristine graphene [193] and rGO [110] evoke an inflammatory response by binding to toll-like receptors (TLRs) and activating the NF-κ B signalling pathway in cells. The NF-κ B signalling cascade is triggered by TLRs and pro-inflammatory cytokines such as IL-1 and TNF-α. Upon activation, NF-κ B shifts from the cytoplasm to the nucleus, facilitating the binding of degrading Iκ B and acting as a transcription factor to synthesize numerous pro-inflammatory cytokines [194]. A schematic of the signalling pathway of TLR4 and TLR9 activated by GFNs is shown in Fig. 5.
Fig. 5
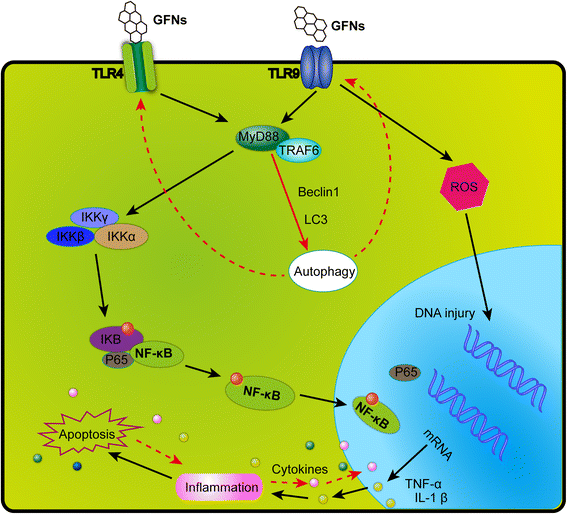
A schematic diagram elucidating signalling pathway of TLR4 and TLR9 responsible for GFNs-induced cytotoxicity. GFNs can be recognized by TLRs, thus activate IKK and Iκ B by a MyD88-dependent mechanism, resulting in the release of NF-κ B subunits and initiating the translocation into the nucleus. Thus, pro-inflammatory factors were transcribed and secreted out of nucleus, modulating the immune responses initiating programmed autophagy, apoptosis and necrosis
|
|
|


