 |
Management of labor and delivery
|
|
|
|
Management of labor and delivery
Following steps must be taken to minimize the chances of feto-maternal bleeding during the time of delivery:
· Delivery should be as nontraumatic as possible. The placenta should not be removed manually to avoid squeezing of fetal cells into the maternal circulation.
· If the manual removal of the placenta is required, it should be performed gently in order to minimize the chances of the feto-maternal bleeding.
· Careful fetal monitoring needs to be performed during the time of labor.
· Umbilical cord should be clamped as soon as possible to minimize the chances of feto-maternal hemorrhage.
· About 15 to 20 cm of cord length should be left intact with the fetus. This may prove useful for exchange transfusion.
· About 5 ml of cord blood should be collected and sent to the lab for tests like ABO and Rh typing; direct Coomb’s test; serum bilirubin levels; hemoglobin estimation and peripheral smear.
· At the time of cesarean section, all precautions should be taken to prevent any spillage of blood into the peritoneal cavity. As far as possible, manual removal of the placenta should not be done.
· A newborn with erythroblastosis should be attended to immediately by a pediatrician who must be prepared to perform an exchange transfusion at once if required.
· In case preterm delivery is required, mother must be administered an intramuscular dose of corticosteroids to increase fetal surfactant level.
After delivery an exchange blood transfusion should be performed when the child has a high level of bilirubin in his blood.
An exchange transfusion involves removing aliquots of patient blood and replacing with donor blood in order to remove abnormal blood components and circulating toxins whilst maintaining adequate circulating blood volume. It is primarily performed to remove antibodies and excess bilirubin in isoimmune disease, the incidence of exchange transfusion is decreasing secondary to the prevention, and improved prenatal management of alloimmune haemolytic disease and improvements in the management of neonatal hyperbilirubinaemia.
The aim of an exchange transfusion is:
• to lower the serum bilirubin level and reduce the risk of brain damage (kernicterus);
• to remove the infants' antibody-coated red blood cells (a source of “potential” bilirubin) and circulating maternal antibodies to reduce red cell destruction;
• to correct anaemia and treat any potential for heart failure whilst maintaining euvolaemia.
Indications for immediate exchange transfusion in haemolytic disease (just after delivery):
• cord Hb < 12 mg/dl and/or
• cord Serum Bilirubin Level (SBR) > 80 µmol/L (or> 5mg/dL).
The procedure is usually performed via an umbilical catheter. Aliquots of blood are removed from the infants and replaced by donor RBCs mixed with plasma. One double volume exchange transfusion is usually effective, but sometimes additional exchange procedures are necessary depending on the degree of postexchange STB rebound.
|
|
|
Rh-negative blood of the same group, must be used for this transfusion, in about 150 ml/kg of the child’s body weight. This procedure helps in correcting neonatal anemia and congestive heart failure. It also helps in removing the circulatory antibodies. The procedure of exchange transfusion involves slow removal of the baby’s blood and its replacement with fresh donor blood or plasma. The blood should be crossmatched with the infant’s serum. The volume of blood to be exchanged is approximately 120-150 ml per 1 kg of body weight.
After this operation the increase of bilirubin should be evaluated hourly. In cases of more than a 8. 55 µmol/l growth of bilirubin during one hour a late exchange transfusion should be performed to the child.
Indications for late (delayed) exchange transfusion in haemolytic disease of a newborn:
• a rate of rise in serum bilirubin of more than 8. 55 - 17. 1 µmol/L/h (0. 5-1 mg/dL/h) is predictive of the ultimate need for late exchange transfusion.
Except for exchange transfusion 10% solution of glucose (8-10 ml/kg of body weight), 10% solution of albumin (5-8 ml/kg of body weight), plasma (8-10 ml/kg of mass) may be administered. Heparin, mannit, antibiotic therapy should be used in addition to phototherapy; vitamin therapy, sedative drugs, symptomatic therapy may be used too.
ABO Blood Group System.
The major blood group antigens A and B are the most common, but are not the most serious cause of hemolytic disease in the newborn. For example, group O women may from early life have anti-A and anti-B isoagglutinins, which may be augmented by pregnancy. Although about 20 percent of all infants have an ABO maternal blood group incompatibility, only 5 percent of them show overt signs of hemolytic disease. Moreover, when they do, the disease almost always is much milder than that with Rh-isoimmunization.
Although ABO isoimmunization will cause hemolytic disease of the newborn, it does not cause hydrops fetalis and is a disease of pediatric rather than obstetrical concern. The reasons for this are at least twofold. First, most species of isoantibodies to A and B antigens are immunoglobulin M, and thus not likely to gain access to fetal erythrocytes. Second, fetal red blood cells have a diminished number of A and B antigenic sites when compared with later in life.
The usual criteria for diagnosis of hemolysis due to ABO incompatibility include the following:
· the mother is blood group O, with anti-A and anti-B in her serum, while the fetus is group A, B, or AB;
· there is onset of jaundice within the first 24 hours;
· there are varying degrees of anemia, reticulocytosis, and erythroblastosis; and
· there has been careful exclusion of other causes of hemolysis.
Unlike the result inRh- hemolytic disease, the Coombs test in ABO incompatibility may be negative, although it is usually positive. Because there is no adequate method of antenatal diagnosis, careful observation is essential in the neonatal period. Unlike Rh- hemolytic disease, ABO disease is frequently seen in first-born infants, and it is likely to recur in subsequent pregnancies. The principles of management of the newborn with ABO hemolytic disease are similar to those for the infant born with Rh-isoimmunization. For simple transfusion or exchange transfusion, group O blood is used. Because the incidence of stillbirths among ABO-incompatible pregnancies is not increased, there is no justification for early labor induction or for performing amniocentesis.
|
|
|
Self Test
1. The incidence of RH-isoimmunization after a full-term delivery in a D-positive infant to an RH-negative mother when no RH immune globulin prophylaxis has been undertaken is
A. 1%
B. 10%
C. 6%
D. 40%
2. An early exchange blood transfusion should be performed if the level of bilirubin is:
A. more than 80 µmol/L
B. more than 102 µmol/L
C. about 34. 6 µmol/L
3. What is the earliest sign of significant fetal anemia on ultrasound examination?
A. enlarged fetal liver
B. polyhydramnios
C. fetal ascites
4. Which of the following is of most importance for development of hemolytic disease of newborn?
A. hemolysis of maternal red cells
B. hemolysis of fetal red cells
5. Which of the following is the most dangerous form of hemolytic disease of newborn?
A. anemia
B. edema
C. enlargement of fetal liver
D. hemolytic anemia with icterus and edema
6. Which form of bilirubin increases at hemolytic disease of the fetus?
A. direct
B. indirect
7. What degree of hemolytic jaundice of the fetus does amniotic bilirubin of 0. 15-0. 24 units mean?
A. 1st degree
B. 2nd degree
C. 3rd degree
D. dead fetus
8. Rh-imune globulin should be given to unsensitized D-negative women in the following cases:
A. at 28 weeks’ gestation to an Rh-negative, nonummunized woman, when the father of the fetus is Rh-positive;
B. at 38 weeks of pregnancy to an Rh-negative woman;
C. before planned pregnancy to an Rh-negative woman.
9. Amniotic bilirubin of 0. 35–0. 7 units means:
A. 1st degree of hemolytic jaundice
B. 2nd degree of hemolytic jaundice
C. 3rd degree of hemolytic jaundice
D. dead fetus
10. If the woman remains nonimmunized and delivers an Rh-positive fetus, Rh-immune globulin is administered:
A. during 24 hours after labor
B. during 2 hours;
C. during 10 days;
D. during one month
CHAPTER 28. BLEEDINGS DURING PREGNANCY AND 1-2 STAGES OF LABOR
Any bleeding from the genital organs in a pregnant woman is called obstetric bleeding.
Obstetric bleedings (haemorrhages) may be classified depending on the term of gestation, reasons, and volume (Table 25).
Table 25
Classification of Obstetric Bleedings
| By term of gestation | By reasons | By volume |
| Early pregnancy | 1. Ectopic pregnancy (cervical, tubal, extrauterine) 2. Spontaneous abortion 3. Missed abortion 4. Artificial abortion 5. Criminal abortion 6. Cervical erosion 7. Cancer of the cervix 8. Bleeding due to varicosis of genital organs 9. Trophoblastic disease | Massive, as a rule |
| Late pregnancy | 1. Premature separation of a normally located placenta 2. Placenta praevia 3. Rupture of the uterus (on the scar area) 4. Hemorrhages because DIC-syndrome (preeclampsia and eclampsia syndrome, dead fetus) | More often massive, but may be of different volume |
| Labor: The 1st stage The 2nd stage The 3rd stage | 1. Premature separation of a normally located placenta 2. Placenta praevia 3. Intrauterine death of the fetus 4. preeclampsia and eclampsia syndrome 5. Water embolism 6. Rupture of the uterus 1. Failure of the process of separation of the placenta from the uterine wall and its expulsion (adherent placenta, fused placenta, defects of the placenta). 2. Injuries of soft tissue of the labor canal (rupture of the cervix, lacerations of mucosal layer of the vagina, rupture of the perineum, rupture of clitoris, vulva, etc. ) | Excessive bleeding, up to hemorrhage shock May be moderate, sometimes massive. As a rule, moderate |
| Early puerperal period Late puerperal period | 1. Hypotonic and atonic bleedings 2. Water embolism 3. Incomplete rupture of the uterus 4. Retention of parts of placenta in the uterus 1. Retention of parts of placenta in the uterus 2. Placental polyp 3. Puerperal endometritis | Massive Moderate more often |
|
|
|
Placenta Praevia
Placenta praevia is an implantation of placenta over or near the internal os of the cervix. The growing placenta thus covers a portion of the lower uterine segment to close (partly or completely) the internal os of the uterus and underlie the presenting part.
The following types of placenta praevia are distinguished:
· Marginal placenta praevia. The placenta does not extend beyond the margin of the internal os. When the cervical canal opens to pass 4-5 cm, smooth membranes and a narrow margin of the placenta can be palpated (Fig. 180).
· Lateral placenta praevia. The placenta covers the 2/3 of the internal os and can be palpated by the examining finger (with the cervix dilated to pass 4 or 5 cm) along almost the entire area except a small portion where the membranes are shown (Fig. 181).
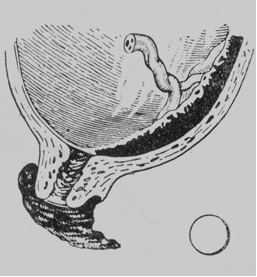
Fig. 180. Marginal placenta praevia
· Total, or central, placenta praevia. The center (or almost the entire central part) of the placenta covers the internal os. When the cervix is dilated to pass 4 or 5 cm, the placenta alone can be palpated (Fig. 182).
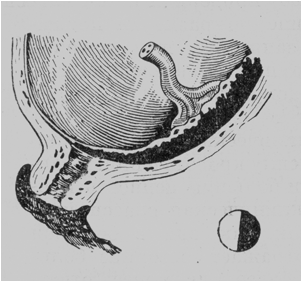
Fig. 181. Lateral placenta praevia
The synonym of total placenta praevia is complete placenta praevia, thus the marginal and lateral one may be named partial placenta praevia.
It is impossible to establish with certainty by the vaginal examination if the center of the placenta lies over the internal os. The diagnosis of central placenta praevia is always stated in cases where the placenta alone can be palpated through the cervix dilated to pass 2 or 3 fingers.
A low attached type is also distinguished (syn.: low-lying placenta). The term low-lying placenta has been used when the placental edge does not reach the cervical os, (Fig. 183) but is close enough to be palpated by an examiner's finger. The current distinction of low-lying placenta, however, does not rely on digital cervical examination. Instead, it is described as a placental edge that approaches to within 2 cm of the cervix on ultrasound examination. The most accurate measurement is obtained by endovaginal scanning. One type of placenta sometimes passes into another during labor. For example, when the internal cervix is only slightly dilated (to pass 2–3 cm) a small margin of the placenta can only be palpated (the marginal placenta praevia), but as the cervix is further dilated, a considerable portion of the placenta becomes palpable, i. e., the marginal placenta praevia turns into the lateral placenta praevia. Thus, finally the diagnosis of type of the placenta praevia may be made after the cervical dilation to 4-5 cm.
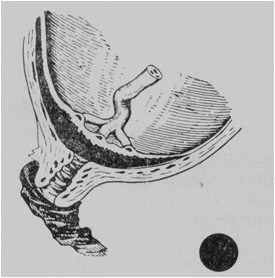
Fig. 182. Total placenta praevia
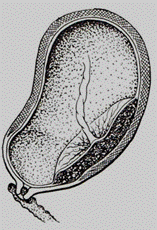
Fig. 183. Low attachment of the placenta (low lying placenta)
Alternative classifications of placenta previa.
Placenta praevia is divided into four grades depending on the relationship and distance to the internal cervical os:
· grade I: low lying placenta: placenta lies in the lower uterine segment but its lower edge does not abut the internal cervical os (i. e lower edge 0. 5-5. 0 cm from internal os).
· grade II: marginal praevia: placental tissue reaches the margin of the internal cervical os, but does not cover it
· grade III: partial praevia: placenta partially covers the internal cervical os
|
|
|
· grade IV: complete praevia: placenta completely covers the internal cervical os
Sometimes types I and II are termed a " minor" or " partial" placenta praevia, and types III and IV are termed a " major" placenta praevia. (Fig184. )
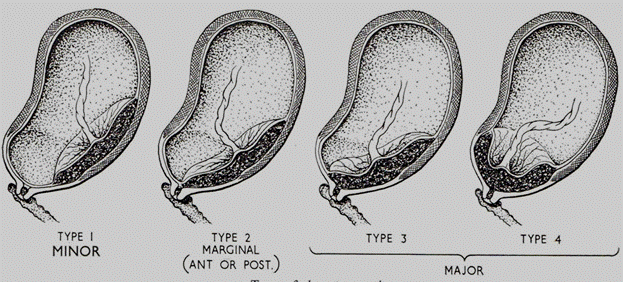
Fig. 184. Types of placenta previa
Sonographic classification of placenta previa is based on distance from the internal os to the placental edge. According to this classification, a placental edge, located 2 cm or more from the internal os is not classified as placenta previa, because it is not associated with intrapartum bleeding. If the placenta is < 2 cm from the internal os it is classified as placenta previa and patient need in further investigations and special management. (Fig. 185)
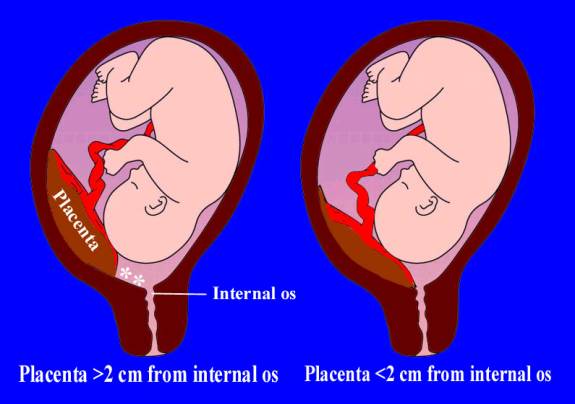
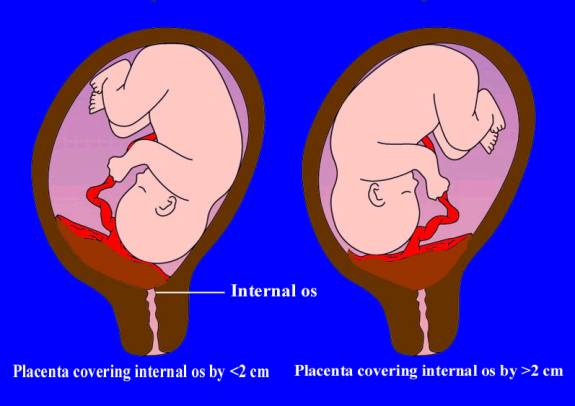
Fig. 185. Types of placenta previa depending on distance between uterine os and the margin of placental tissue
Etiology
Inflammatory, atrophic and other pathological processes favour the implantation of the egg in the lower uterine segment and the development of placenta praevia. When the endometrium is affected by pathology, the conditions for normal implantation are impaired and the egg descends to the lower uterine segment, and implants near the internal os. Placenta praevia may develop as a result of septic or gonorrheal endometritis; abortions, especially recurring, and abortions complicated with inflammatory diseases also predispose the patient to placenta praevia. Chorionic villi may develop over a considerable area and occupy the region adjacent to the deciduas basalis, extending over the region of the deciduas capsularis. This propagation of the chorionic villi will stimulate the growth of the placenta to reach the internal os and cover it.
The condition is unfavourable for both the mother and the fetus. The central (total) placenta praevia is especially dangerous.
The blood vessels of the lower uterine segment in patients with placenta praevia are markedly dilated, elongated, and contain much blood. The uterine cervix is richly permeated with blood vessels so that the cervical tissue becomes very much like cavernous. It is now very soft and can be easily injured by careless manipulations. The broken vessels begin bleeding and the hemorrhage may become profuse and fatal to the woman. In the later months of pregnancy the lower uterine segment widens to accommodate the lower pole of the fetus with the presenting part. The wall of the lower uterine segment is distended and misaligned with the placental surface so that parts of the placenta are separated from the uterine wall and the uteroplacental vessels thus break and begin bleeding.
Bleeding in placenta praevia may begin in late pregnancy and intensify during labor. Hemorrhage sometimes begins with the onset of regular contractions. Due to muscular retraction the lower uterine segment and the margins of the cervical os are pulled off from the placental surface to cause its separation from the site of attachment. The stronger the uterus contracts and the wider the cervix opens, the higher the degree of placental separation will be. The blood vessels break in the region of the placental separation and fatal bleeding may sometimes develop. The fetal blood circulation is not however affected since these are the maternal vessels that break, but the fetus still can develop asphyxia due to the decreased area of the placenta involved in the gas exchange of the fetus. Prognosis for the fetus in placenta praevia is always aggravated because an operation may often be necessary and this adds to danger.
Placenta praevia favours the development of infectious diseases. Blood coagulated at the site of placental separation is a good nutrient medium for the propagation of microbes that may enter from the vagina. Anemia, which weakens the body resistance to infection, is also harmful.
The villi of placenta praevia can sometimes grow very intensely to complicate the course of the placental stage of labor (the separation of the placenta is delayed and blood loss increases).
Incidence
Placenta praevia occurs in 1 of 200 deliveries. This pathology is more likely to occur in multiparas, in patient who have had a cesarean section, or in patients with uterine abnormalities (post-abortion inflammatory diseases in past history, fibroid) that inhibit normal implantation. The total (central) placenta previa occurs much less frequently than the partial (marginal or lateral) placenta praevia.
|
|
|


