 |
Preparation of the analyzer for work
|
|
|
|
Install the analyzer on the horizontal plane, having provided convenience of work and a condition of natural ventilation. Install the sample receptacle in the holder. Connect the power supply unit to network ~ 220 V or a cable for food from onboard network of the car to the car lighter 12 V. Connect the output connector of the power supply unit (or a cable for food from onboard network of the car) to the socket of connection of the power supply of the analyzer 1. On the display the message will appear:

In 2 seconds in the lower line of the display serial number of your analyzer, and then number of the current analyzed product (number of graduation) appears. The analyzer will include the mode of warming up and on the display the message will appear:

On warming up of the analyzer no more than 5 minutes will leave.After warming up on the display to appear the message:

The entrance to the service menu is carried out as follows: press at the same time the INPUT and START buttons, then release the START button, continuing to hold the ENTER button until on the indicator the inscription appears:

Press the button <or> for the choice of a menu item.
The menu consists of two points:
1. Choice of a product (graduation choice). If your analyzer calibrated for measurement more than one type of products (or has several options of graduation), you can choose the necessary product (one of five).
2. Language of messages. In this point it is possible to change language of the messages displayed on the display (Russian/English). During the work with the menu the following buttons are used:
"INPUT" - confirmation of the selected item.
<or> - the choice of the necessary point.
For an exit from the menu press the START button.
2.4 Use of the analyzer
Make sure that the analyzer is ready to work - on the display the message has to be displayed:</or></or>

Pour test in a probopriyemnik. The analyzer will begin measurement automatically. On the display the message will appear:

During measurement the lower line of the display will be filled with rectangles. Process of measurement will come to the end when all lower line is filled with rectangles. After the end of measurement on the display results will appear:

Where: F– fat; With - SOMO; In W– water; П – density.
Washing
• disconnect a food cord from network;
• warm up clear water to 50-60 °C and part in it the Reactant No. 1 (0,5g. on 100 ml. waters);
• pour into a sample receptacle of this solution (wash solution № 1);
• connect the syringe for washing to the union and make the piston of the syringe of 10-15 back and forth motions;
• replace flushing solution and repeat washing;
• change water until water doesn't become transparent;
• wash out the measuring channel of the analyzer the distilled water;
• blow the channel the empty syringe.
Report on performance of a task
By results of tests write a conclusion.
|
|
|
Control questions:
1. What it is based analysis BONDS methods on?
2. What parameters measure BONDS methods?
3. What requirements are imposed to the made milk on physical and chemical indicators?
Laboratory work № 3
Studying of methods of definition of a mass fraction of protein in milk and dairy products
Work purpose: To get acquainted with various methods of definition of protein in milk.
Task:
1. To study various methods of definition of protein in milk.
2. To give the comparative analysis of the received results, to specify the reasons of divergences of values of a mass fraction of protein when determining by various methods.
3. To draw a conclusion on expediency of use of the studied methods in laboratory and industrial scales.
Short theoretical data
Definition of albumens in milk by method of formal titration. The method is based on binding of free amino groups of proteins by aldegidny groups of formalin therefore acidity of milk changes. The shift (on a titration curve) to the area of lower values рН is observed. The method is applicable for determination of the sum of albumens in milk (acidity not higher than 220 0T).
Definition of percentage of protein in milk by a refraktometrical method. The index of refraction of Pd of milk consists of the sum of Pd of water and Pd of the proteins dissolved in her, dairy sugar, nonprotein nitrogenous substances and salts. Nonprotein nitrogenous substances, dairy sugar and salts are in milk in the form of true solution, a squirrel - in the form of colloidal. Fat in milk is in a type of an emulsion and doesn't influence total index of refraction of milk. Content of proteins (the sum of casein, albumine, globulin) in milk is determined by a difference of indications on a scale of "Squirrels" of milk and bezbelkovy serum under identical conditions of the performed measurements.
Colorimetric method of definition of protein in milk. The method is intended for the research purposes. Ability of proteins to connect sour dyes is the basis him. By a colorimetric method, as well as by a standard method of Kjeldal, define a mass fraction of the general protein, including fraction of nonprotein nitrogen.
Express measurement of protein content in milk on devices a belkomer of BMTs-1 or BM-003. The principle of operation of the device is based on a photocolorimetric method of measurement of optical density of the filtered mix of a dose of milk and a dose of solution of dye of organic acid blue-black.
The method is based on ability of proteins of milk at рН below an isoelectric point to connect sour dyes, forming with them an insoluble complex, at the same time the optical density of solution of dye decreases in proportion to amount of protein. After removal of an insoluble complex measure again the optical density of the solution containing ostatochno untied dye which is proportional to a mass fraction of protein in milk. Structurally the device consists of two blocks: measuring and block of preparation of test.
Definition of the general protein in milk by Kjeldal's method. The method of definition of nitrogenous substances in foodstuff has been offered by Kjeldal in 1883 and still, with little changes, is the main method of definition of albumens in milk and dairy products. Kjeldal has developed a method of determination of amount of proteins on nitrogen, bases in milk and dairy products. Kjeldal has developed a method of determination of amount of proteins on nitrogen, that organic substances when heating with the concentrated sulfuric acid are oxidized to H2O and CO2, and nitrogen of amino groups – NH2 passes into sulfate ammonium.
|
|
|
Sulfuric acid when heating decays on sulphurous anhydride, atomic oxygen and water:
H2SO4 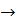 SO3 + H2O SO2 + O + H2O
SO3 + H2O SO2 + O + H2O
The emitted atomic oxygen oxidizes amino acids. After the end of oxidation aminny nitrogen of albumens will be in a form of ammoniyny salt. Transferring nitrogen of proteinaceous connections to a form of sulfate ammonium, define amount of nitrogen in the form of ammonia, for this purpose dilute solution with water, neutralize sulfuric acid solution of a caustic natr and surplus create him alkaline reaction thanks to what ammoniyny salts emit ammonia:
(NH)2SO4 + 2NaOH = Na2SO4 + 2NH3 + H2O
The formed ammonia is overtaken in a reception flask with boric acid, Borat of ammonium which in the water environment is strongly hydrolyzed as a result turns out and has the alkaline environment, For neutralization he is used hydrochloric acid. By the number of ml of 0,1 N of HCl solution who have gone for titration borathat find amount of nitrogen. For determination of amount of proteins the amount of nitrogen is multiplied by coefficient 6,38. The coefficient is established from calculation that the amount of nitrogen is equal in milk proteins to 15,65%.
Equipment, devices and technical means:
The refractometer, bath water, scales, pipettes on 1 ml, 5 ml, 10 ml, 20 ml, the device for measuring off of formaldehyde with a capacity of 1 ml, a test tube with a capacity of 10 ml, a flask measured on 100 ml, filter paper, Kjeldal's flask, a byuksa with a cover, acetic acid 10% solution, calcium chloride 4% solution, фенолфталеин 2% solution, a hydroxide of sodium of 0,1 N solution, formalin 36-40% solution, cobalt sulfate 25% solution, milk of different structure; sulfate potassium, mercury oxide, sulfate copper, sulfuric acid, Tashiro's indicator, boric acid.
Work performance order
Definition of albumens in milk by method of formal titration. Bring in a glass with a capacity of 150-200 ml a pipette 20 ml of milk, 0,25 ml of 2% solution of a fenolftalein and titrut 0,1 N sodium hydroxide solution before emergence of the weak-pink coloring corresponding to a control standard. Then bring 4 ml of the neutralized 36-40% formaldehyde and again titrut before such coloring, as well as at the first titration.
For preparation of a control standard of coloring in the same glass measure 30 ml of milk and 1 ml of 0,25% - го cobalt sulfate solution. The standard is suitable for work during one change. In order to avoid a sediment of cream the standard is recommended to be poured periodically. The share of the general protein in milk can be determined also by table 3.1
Table 3.1
| Number of 0,1 N of NaOH solution, см3 | Mass fraction of proteins in milk, % | Number of 0,1 N of NaOH solution, см3 | Mass fraction of proteins in milk, % |
| 2,45 2,50 2,55 2,60 2,65 2,70 2,75 2,80 2,85 2,90 2,95 3,00 3,05 3,10 3,15 3,20 3,25 | 2,35 2,40 2,44 2,49 2,54 2,59 2,64 2,69 2,73 2,78 2,83 2,88 2,93 2,98 3,03 3,07 3,12 | 3,30 3,35 3,40 3,45 3,50 3,55 3,60 3,65 3,70 3,75 3,80 3,85 3,90 3,95 4,00 4,05 4,10 | 3,16 3,21 3,25 3,31 3,35 3,40 3,45 3,50 3,55 3,60 3,65 3,69 3,74 3,79 3,84 3,89 3,94 |
In parallel make check experiment on neutralization of mix of 20 ml of water and 5 ml of solution of formaldehyde.
Processing of results.
The mass fraction of protein (X2) is calculated as a percentage on a formula:
X2 = (V2 - V1 - V0) * 0,96 + X1, (1)
|
|
|
where: V2 - the total of solution spent for neutralization, ml; V1 - the amount of solution spent for neutralization before introduction of formaldehyde, ml;
V0 - the amount of solution spent for check experiment, ml;
0,96 - empirical coefficient, %/ml;
X1 - the amendment to result of measurement of a mass fraction of protein, %.
Determine the percentage of protein in milk by the refractometric method. For receiving protein-free serum measure 5 ml of the studied milk in a bottle and add to him 5-6 drops of 4% solution of chloride calcium (40 g of CaCI2 in one liter of the distilled water).
The bottle is closed a stopper and slightly shake up contents. At the same time prepare 2-3 parallel tests (bottles have to be numbered). Bottles place in a tank, pour in it water to a half of height of bottles, close a cover. Water in a tank is boiled within ten minutes. Then hot water is replaced cold. Bottles cool within two minutes. Bottles take out from a tank, stir up so that the clot has collapsed and the emitted serum has mixed up with condensate.
The filtered serum is applied on a measuring prism of the refractometer and smoothly close it a lighting prism. Observing in an eyepiece, clean an color of border of a treatment of light and shade. For improvement of sharpness of border measurement needs to be taken in 0,5 - 1 min. since during this time from test air is removed and the surface of a lighting prism is better moistened.
On a scale of "Squirrels" read the instrument for serum. Measurements repeat 3-4 times and count arithmetic-mean value of Bs. Having removed serum from both prisms, they are carefully washed out water and wiped a pure soft napkin or cotton wool. Then 1-2 drops of the studied milk place on a measuring prism.
Take measurements on a scale of "Squirrels" in the same order, as on serum. As sharpness of border at milk is slightly worse, than at serum and water, measurements repeat 4-5 times and count arithmetic-mean value of Bm. Content of proteins in milk is determined by a formula:
Bmol. % = Bm – Bs (2)
The general protein (proteins and nonprotein nitrogenous substances) is determined by a formula:
Бо.б. = (Bm - Bs) *1,0855 (3)
It is also possible to determine the content of casein, serumal proteins (albumine and globulin) in milk by a scale of "Squirrels". Content of casein in milk is determined by a formula:
To % = (Bm - Bk.s.)*1,1012, (4)
where Bk.s. – the indication on a scale of "Squirrels" for casein free serum.
For receiving casein free serum with milk (5 ml) bring 10 drops of 10% solution of acetic acid in a bottle. Content of serumal proteins is determined by a formula:
% SB = Bm – K (5)
Definition of the general protein in milk by Kjeldal's method. Place in Kjeldal's flask consistently several glass beads or pieces of porcelain, about 10 g of sulfate potassium, 0,5 g of an oxide of mercury or 0,04 g of sulfate copper. In a bottle with a cover measure 5 ml of milk, close a cover and weigh with an accuracy of 1 mg. The empty bottle is weighed again and on a difference between the mass of a bottle with milk and mass of an empty bottle establish the mass of the taken milk. Add 20 ml of sulfuric acid to a flask, pouring carefully on flask walls, washing away from them milk drops. The flask is closed a pear-shaped glass stopper and carefully roundabouts mix flask contents.
The flask is put on the heating device in inclined situation at an angle 450 and carefully heated. Continue heating of a flask until foaming stops and contents of a flask don't become liquid. Then burning is continued at more intensive heating. Extent of heating is considered sufficient when the boiling acid is condensed in the middle of a mouth of a flask of Kjeldal.
|
|
|
From time to time contents of a flask are mixed, washing away the charred particles from flask walls. Heating is continued until liquid doesn't become absolutely transparent and almost colourless (at application as the mercury oxide catalyst) or slightly bluish (at application as the catalyst of sulfate copper).
After solution clarification heating is continued during 1,5 h then to a flask allow to cool down to room temperature. Add 150 ml of the distilled water and several pieces of svezheprokalenny pumice, mix and again cool.
In a conic flask measure 50 ml of solution of boric acid, add 4 drops of the indicator of Tashiro and mix. The conic flask is connected to the refrigerator by means of the allonge and a rubber stopper so that the end of the allonge was below the surface of solution of boric acid in a conic flask. Kjeldal's flask is connected to the refrigerator by means of the drop catcher passing through one stopper with a delitelny funnel. The graduated cylinder measure 80 ml of solution of a hydroxide of sodium (at application as the catalyst of a red oxide of mercury use the sodium hydroxide solution containing sodium sulfide) and through delitelny (or drop) a funnel bring him in Kjeldal's flask. At once after pouring out of solution close the crane of a delitelny funnel for avoidance of loss of the formed ammonia. Contents of a flask of Kjeldal are carefully mixed roundabouts and heated to boiling. At the same time it is necessary to avoid foaming.
Continue distillation until liquid doesn't begin to boil pushes. At the same time regulate extent of heating so that time of distillation was no more than 20 min. It is possible to be convinced of completeness of distillation of ammonia by additional distillation in a new consignment of boric acid (20 ml) within 5 min. Coloring of solution of boric acid has to be left without change. At distillation don't allow heating of solution of boric acid in a conic flask. Too strong cooling (lower than +10 0C) is also undesirable as it can cause transfer of liquid from a conic flask in Kjeldal's flask.
Before the end of distillation lower a conic flask so that the end of the allonge has appeared over the surface of solution of boric acid, and continue distillation within 1-2 min. Stop heating and disconnect the allonge. In a conic flask wash away external and internal surfaces of the allonge a small amount of the distilled water. Titrut distillate of 0,1 N solution of hydrochloric acid before transition of green color to violet.
In parallel carry out the control analysis as well as the main, applying 5 ml of the distilled water instead of milk. The control analysis is carried out in each series of determination of amount of protein and at each replacement of reactants.
The mass fraction of the general protein (B) is calculated as a percentage to within the third decimal sign on a formula:

where 1,4 – amount of nitrogen, equivalent 1 ml 0,1 N of solution of hydrochloric acid, mg;
0,1 – normality of solution of hydrochloric acid;
V1 – the volume of 0,1 N of the hydrochloric acid spent for titration of distillate in the main analysis, ml;
V0 - the volume of 0,1 N of the hydrochloric acid spent for titration of distillate in the control analysis, ml;
6,38 – coefficient for transfer of a mass fraction of the general nitrogen to a mass fraction of the general protein;
m – the mass of the milk taken on the analysis.
Report on performance of a task To present results of work in the form given in
tab. 3.2
| Research methods | Mass fraction of protein, % |
| Method of formal titration Refraktometrichesky Kjeldal's method |
Control questions
1. Proteins of milk, their classification.
2. Methods of formal titration and Kjeldal, their chemical essence.
3. The Refraktometrichesky method on what properties of casein and serumal proteins it is based?
Laboratory work № 4
|
|
|


