 |
Studying of methods of definition of a mass fraction of lactose and mineral substances
|
|
|
|
Work purpose: to get acquainted with methods of definition of lactose and mineral substances in milk.
Task:
1. To study methods of definition of lactose and mineral substances in milk.
2. To define the following indicators in the studied samples: mass fraction of lactose and mass fraction of calcium.
3. To analyse the received results, to draw a conclusion on lactose, what of methods of definition, it is more expedient to apply to laboratory and industrial control.
Short theoretical data
Determination of content of lactose by a refraktometrichesky method. Refractometry — determination of index of refraction, and refraction number – the conditional number showing refraction size in terms of a scale of this refractometer. The ray of light passing through various environments deviates the rectilinear way on a bigger or smaller corner depending on properties of the environment through which he passes.
Definition of a mass fraction of lactose by a polarimetric method (according to G. Vizhinayta). Existence in molecules of sugars, in particular, of lactose, asymmetric carbon atoms does them optically active, that is capable to rotate the plane of the polarized light. This property is function of concentration of water solutions of carbohydrates therefore, measuring an angle of rotation α, it is possible to define concentration of lactose. Solutions for polarimetric researches have to be transparent therefore apply acetic zinc and zhelezistosinerodisty potassium to sedimentation of proteins and fat.
Determination of calcium complexometric method (byDudenkov).The method is based on instant education the slabodissotsiirovannykh of complex connections of various cations with trilonom-B (disodium salt of etilendiamintetrauksusny acid).
Various metals, irrespective of valency, react with trilonom-B in the molar relation 1:1. All intra complex connections are very steady also rastvorima in water. When determining calcium use the indicator mureksidy. Mureksid forms complex connection of intensively red color with calcium. However this connection is less steady, than complex compound of calcium with Trilonom-B. Therefore at complexometric titration in an equivalence point cations of calcium disappear and there is a sharp change of coloring of solution in lilac to a bluish shade. Solutions, titrable trilonom-B with application of a mureksid, alkalinize a caustic natr to рН not lower than 12.
Equipment, devices and technical means:
Refractometer, bath water, SU-4 saccharimeter; calcium chloride 4% solution, sodium hydroxide 8% solution, trilon-B 0,1n solution, calcium 0,1n chloride solution, indicator мурексид, solutions of acetic zinc and zhelezistosinerodisty potassium, solution of bromnovatokisly potassium 0,2n; pipettes of 5 ml, test tubes on 10 ml, flasks conic with a capacity of 200-250 ml, 100 ml, flasks measured with a capacity of 100 ml, cylinders measured, burettes.
Work performance order
Determination of content of lactose by a refraktometrichesky method. Measure a pipette in a test tube 5 ml of the studied milk, add 5-6 drops of 4% solution of chloride of calcium. Test tubes close traffic jams and place in a water bath with the boiling water for 5 min., then cool them to 15 °C, at the same time pay attention to that drops of the condensed water didn't remain on test tube walls. Then open a stopper and carefully extend serum in a glass tube which lower end is closed by cotton wool for a serum filtration. The drop of transparent serum is applied on a surface of the lower prism of the refractometer and slowly lower the top prism and take measurements.
|
|
|
The mass fraction of dairy sugar in milk is found according to tab. 4.1
Table 4.1 - Dependence of a mass fraction of lactose in milk from index of refraction
| Index of refraction at 17,5°С | Mass fraction of dairy sugar, % | Index of refraction at 17,5°С | Mass fraction of dairy sugar, % | Display -tel prelomle-niya at 17,5°С | Mass fraction dairy sugar, % |
| 1,3390 1,3400 | 3,01 3,06 3,11 3,16 3,21 3,26 3,31 3,36 3,42 3,47 3,52 3,57 3,62 3,67 3,70 | 1,3405 | 3,72 3,77 3,82 3,78 3,93 3,98 4,03 4,08 4,13 4,18 4,23 4,28 4,33 4,38 4,44 | 1,3420 | 4,49 4,54 4,59 4,64 4,69 4,74 4,79 4,84 4,89 4,95 5,00 5,05 5,10 5,15 5,20 |
Definition of lactose in milk by the accelerated method. To neutralize the filtrate received in the previous experience sodium hydroxide solution before alkalescent reaction on the indicator a fenolftaleina. To halve a filtrate. In a pure test tube to pour 2 ml of solution of sodium hydroxide and on drops to add when stirring solution of sulfate of copper (II) before emergence of a deposit of hydroxide of copper (II) of blue color. The received mix to shake up and add the first half of a filtrate. Emergence of bright blue coloring indicates presence at a lactose filtrate. When heating coloring of solution changes till red color. To evaporate the second part of a filtrate in a porcelain cup. Education on walls of a cup of a brown raid with a characteristic caramel smell indicates lactose presence.
Definition of a mass fraction of lactose by a polarimetric method (according to G. Vizhinayta). In a glass on 100 ml weigh 33 g of milk with an accuracy of 0,01 g. Milk is quantitatively transferred to a measured flask with a capacity of 100 ml, rinsing a glass with the distilled water. In a flask flow 5 ml of acetic zinc and zhelezistosinerodisty potassium. Flask contents after addition of each reactant are carefully mixed, without stirring up in order to avoid formation of vials of air. Then flow 25 ml 0,2n solution of bromnovatokisly potassium, carefully mix.
Contents of a flask are brought the distilled water to a tag and carefully mixed, strongly stirring up. In 5-10 min. filter via the dry folded filter in a dry flask. The filtrate is polarized in a polarimetric tube 400 mm long. Counting is carried out by 3-5 times and take arithmetic-mean. Content of lactose is calculated on a formula:
L=  , (1)
, (1)
where P — the indication of a saccharimeter;
K — the amendment on deposit volume.
Calcium definition by a kompleksonometrichesky method (across Dudenkov). To 5 ml of milk in a chemical glass flow 90-95 ml of the distilled water, 5 ml of 8% NaOH solution and from the burette measure precisely 3,5 ml 0,1n solution trilona-B, mix a glass stick and leave for 2 min. Bring about 0,04 g of dry mix of the indicator of a mureksid with chloride sodium on a knife tip (1 part of a mureksid carefully pound with 50 parts of chemically pure chloride sodium). Solution is painted in lilac color. Contents are titrut 0,1n CaCI2 solution, adding him on drops at continuous hashing before emergence of steady pink coloring. Then from the burette flow when stirring on drops solution trilona-B before emergence of steady lilac color again. In 1 min., in case of coloring disappearance, add 1 more drop Trilona-B.
|
|
|
From the total amount of solution trilona-B, spent for the first and second titration and counted on precisely 0,1n solution, subtract volume 0,1n the CaCI2 solution spent for the return titration and find volume trilona-B, the milk connected with calcium. Content of calcium in mg of % is calculated by a formula:
Ca= 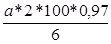 , (2)
, (2)
where and – quantity 0,1n solution trilona-B, connected with calcium, ml;
2 amount of calcium, corresponding 1 ml 0,1n solution trilona-B, mg;
5 – the volume of the milk taken for the analysis, ml.
|
|
|


