 |
Cordocentesis
|
|
|
|
The achievements of modern medicine can not only reveal some serious diseases even in unborn man, but also to carry out his treatments.
Such methods are called " prenatal" and use for medical reasons in relation to the unborn child. For the purpose of prenatal diagnostics and prenatal therapy cordocentesis performed.
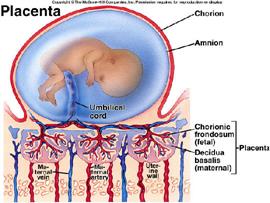
Fig. 90. The structure and function of the placenta.
Cordocentesis ( CC, also sometimes called Percutaneous Umbilical Cord Blood Sampling - PUBS) – is an invasive method utilized in prenatal diagnostics. The ultrasound guides a needle through the mother's abdomen and uterine wall into the fetal vein of the umbilical cord in order to retrieve a sample of fetal blood. The collected fetal lymphocytes can be then utilized for karyotype or molecular genetic analysis in order to exclude chromosomal aberrations or genetic diseases in the fetus. The fetal blood represents precious diagnostic material that can be used further for e. g. determination of blood group of the fetus, alloimunization or infection diagnosis. The risk of cordocentesis is, when performed by an experienced physician, comparable to that of amniocentesis (the risk of miscarriage is below 1%).
Cordocentesis became wide spread in the 2nd half of 20th century, when it became possible to mass adoption ultrasound devices in medical centers (Fig. 90).
Cordocentesis is most often used for the diagnosis of fetal diseases, such as:
• Down's Syndrome, Edwards Syndrome, Patau's syndrome (trisomy 21, 18, chromosome 13, respectively);
• Shereshevsky turner syndrome (monosomy X);
• Klinefelter's syndrome (two or more extra X chromosome in addition to a Y chromosome);
• Duchenne muscular dystrophy (DMD) is a genetic disorder characterized by progressive muscle degeneration and weakness. DMD has an X-linked recessive inheritance pattern and is passed on by the mother, who is referred to as a carrier.
• Cystic fibrosis is an inherited disease characterized by the buildup of thick, sticky mucus that can damage many of the body's organs. The disorder's most common signs and symptoms include progressive damage to the respiratory system and chronic digestive system problems. The features of the disorder and their severity varies among affected individuals.
• Hemophilia is a rare hereditary disease associated with impaired coagulation (blood clotting); the diseases characterized by bleeding into joints, muscles and internal organs, both spontaneous and as a result of injury or surgery. In hemophilia dramatically increases a patient's risk of death from bleeding in the brain and other vital organs, even a minor injury. Patients with severe hemophilia, are disabled because of frequent bleeding into joints (hemarthrosis) and muscle tissue
• (hematoma). There are two types of hemophilia (A, B). Hemophilia A is an X-linked, recessive disorder caused by deficiency of functional plasma clotting factor VIII (FVIII), which may be inherited or arise from spontaneous mutation. The development of inhibitory antibodies to FVIII can result in acquired hemophilia A or can complicate the treatment of genetic cases. Hemophilia A is the most common type of hemophilias. Hemophilia B, or Christmas disease, is an inherited, X-linked, recessive disorder that results in deficiency of functional plasma coagulation factor IX. Spontaneous mutation and acquired immunologic processes can result in this disorder as well. Hemophilia B constitutes about 20% of hemophilia cases, and about 50% of these cases have factor IX levels greater than 1%.
|
|
|
The thalassemias are inherited disorders of hemoglobin (Hb) synthesis. Thalassemia is a disease with many forms, all of which are characterized by impaired production of one of the normal globin peptide chains found in HB. Healthy adults should have more than 95% hemoglobin A (HbA), which consist of 2 alpha and 2 beta peptide chains. Other polypeptide chains are gamma, delta, epsilon, and zeta. The synthesis of globin chain is partially or completely suppressed resulting in reduced Hb content in red cells, which then have shortened life span. There are two primary types of Thalassemia disease: Alpha Thalassemia disease and Beta Thalassemia diseases. Both may be in major and minor forms. Alpha thalassemia results in an excess of beta globins, which leads to the formation of beta globin tetramers (β 4) called hemoglobin H. These tetramers are more stable and soluble, but under special circumstances can lead to hemolysis, generally shortening the life span of the red cell. Conditions of oxidant stress cause Hgb H to precipitate, interfering with membrane function and leading to red cell breakage. The most severe thalassemia is alpha thalassemia major, in which a fetus produces no alpha globins, which is generally incompatible with life. Beta thalassemia results in an excess of alpha globins, which leads to the formation of alpha globin tetramers (α 4) that accumulate in the erythroblast (immature red blood cell). These aggregates are very insoluble and precipitation interferes with erythropoiesis, cell maturation and cell membrane function, leading to ineffective erythropoiesis and anemia. Children born with beta thalassemia major (Cooley anemia) are normal at birth, but develop severe anemia during the first year of life. Other symptoms can include: bone deformities, growth failure, yellow skin (jaundice), iron overload in their bodies, either from the disease itself or from frequent blood transfusions, infections, Enlarged spleen, Heart problems, and anemia.
• Intrauterine infection of the fetus;
• determination of acid-base balance of the fetus as an indicator of intrauterine fetal growth retardation, to identify the causes of the pathological state;
• therapeutic transfusion (when autoimmune, alloimmune conditions);
• fetal drug therapy.
Analysis of a fetus’ blood allows to obtain the maximum, compared with other diagnostic studies, amount of information about the fetus’ condition and development, and eliminate erroneous results, for example, associated with mosaicism of the placenta.
However, the complexity of its implementation, related to the mobility of the umbilical cord of the fetus, determination of the optimal location of the puncture, etc., is not allowed to suggest cordocentesis priority as a method of invasive diagnostics, as there are less complicated and safer procedures (chorionic villous sampling, amniocentesis).
|
|
|
Puncture of the umbilical cord vessels can be performed only starting from the 18th gestational week, and it is better, at 21 – 24th week of pregnancy. (Fig. 91)

Fig. 91. Cordocentesis procedure


Fig. 92. The fetal blood sampling (cordocentesis).
Technically cordocentesis performed about the same as other similar invasive procedures during pregnancy, using the method of " free hand" (in most cases) or using the needle adapter (Fig. 92).
Single-needle method or the two-needle puncture are used in practice:
• In the first case, puncture of the amniotic SAC, puncture of the vessels of the umbilical cord of the fetus produced by the same needle, then needle attached to the syringe, the necessary amount of fetal blood ( 1-4 ml) aspirated, and the needle removed in reverse order;
• In the two – needle method puncture of the umbilical cord produced by a small needle, which is located inside the outer needle of larger diameter. With a larger needle first aspiration of the amniotic fluid (amniocentesis) preformed, if necessary, and then –puncture of an umbilical vein and aspiration of cord blood with a small needle is performed. After successful aspiration of material in the reverse order of the needle removed from the uterus.
The operation is performed under ultrasound control, and CTG control in late pregnancy (in third trimester).
When properly performed, the accuracy of the results is increased to 99. 9%.
|
|
|


